Abstract
Introduction : Approximately 40% of patients with diffuse large B-cell lymphoma (DLBCL) who receive standard therapy relapse or develop refractory disease, have poor clinical outcomes (median overall survival of 6 months) with subsequent salvage therapies (Sehn LH. Hematology Am Soc Hematol Educ Program. 2012; Crump, et al. ASCO 2016). The novel cereblon modulating agent CC-122 promotes ubiquitination and degradation of hematopoietic transcription factors Ikaros and Aiolos in a cereblon-dependent manner, resulting in both DLBCL cell autonomous activity as well as immunostimulatory effects. CC-122 induced Aiolos and Ikaros degradation leads to increased apoptosis in both activated B-cell (ABC) and germinal center B-cell (GCB) DLBCL cell lines, in contrast to the ABC subtype selectivity of lenalidomide (Hagner P et al. Blood. 2015).
The maximum tolerated dose of CC-122, active ingredient in capsule (AIC) was established for continuous oral dosing at 3 mg AIC daily (QD) in multi-tumor cohorts (Gandhi A, et al. Blood. 2013). Subsequently, intermittent dosing schedules were evaluated in lymphoma; 4 mg AIC taken 5 out of 7 days (5/7) was chosen for further expansion (Carpio C, et al. Blood 2015 126:1494). Finally, a formulated capsule was introduced, CC-122 (F6); the recommended phase II dose was chosen based on pharmacokinetics and tolerability (3 mg, 5/7).
Methods : Subjects received oral doses of CC-122 AIC on continuous (28/28) or intermittent (21/28 or 5/7) schedules, or CC-122 F6 on the 5/7 schedule. Key inclusion criteria include age ≥18 years, and relapsed or refractory disease, defined as progression following (or unable to tolerate) ≥1 prior anthracycline or alkylating agent containing regimen (with or without anti-CD20). Adverse events (AE) were evaluated per NCI CTCAE v4.03 and efficacy by 2007 IWG Criteria. DLBCL cell-of-origin and a novel gene expression classifier representing tumor microenvironment content were tested using NanoString on tumor biopsies and correlated to efficacy.
Results:As of May 1, 2017, 97 R/R DLBCL subjects from 18 institutions were enrolled/treated; 81 were enrolled at doses/schedules selected for expansion (3 mg QD AIC n=24, 4 mg 5/7 AIC n=39, and 3 mg F6 5/7 n=18), and 16 were enrolled at other dose levels. Eight subjects were ongoing as of the data cutoff date. Twelve subjects had transformed lymphoma (12.4%) and 85 DLBCL, not otherwise specified (NOS) (87.6%). The median age was 62 (36% were >65) and 57% were male, 98% had ECOG 0-1 at screening, 62% were refractory to their last prior therapy (SD/PD), 30% had CR to prior R-CHOP, and 44% had <CR to prior R-CHOP. Median prior lines was 3 (range 1-13), 86% were treated with ≥2 prior lines of therapy.
Of the 57 R/R DLBCL subjects enrolled in the 5/7 schedule at doses chosen for expansion, a total of 46 subjects (81%) experienced ≥1 Grade 3-4 treatment emergent adverse event (TEAE), of which 34 (60%) was suspected to be related to CC-122, and serious adverse events (SAE) suspected to be related to CC-122 were reported in 25% of subjects. The most common grade 3-4 TEAEs were neutropenia (39%) and infections (combined term, 17.5%). There was 1 grade 5 event of pneumonia suspected to be related to CC-122. Dose reduction due to AEs (most commonly due to neutropenia) occurred in 12 subjects (21.1%); 40.4% of subjects received at least one dose of G-CSF. Fifty-one subjects (89%) had discontinued treatment as of the data cutoff; the most common reasons for study discontinuation were disease progression (60%), adverse event (16%), death (5%), and candidate for transplant (4%).
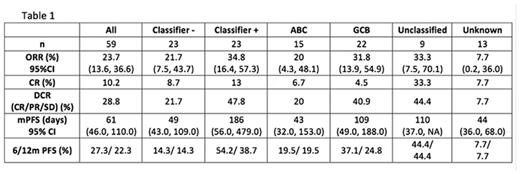
Pooled efficacy is shown for DLBCL NOS subjects with at least 2 prior lines of therapy treated at doses/schedules of CC-122 selected for expansion. As observed in Table 1, gene classifier positive patients had a better outcome than gene classifier negative patients
Conclusions:CC-122 appears well tolerated with encouraging activity in patients with R/R DLBCL. CC-122 has broad activity across cell of origin including in ABC, GCB, and unclassified subsets. Preliminary efficacy data demonstrated differential effects of CC-122 between gene classifier positive and negative subjects. A novel gene expression signature is being established to enrich for responders to CC-122. The observed signals of efficacy are encouraging in this R/R DLBCL subset. CC-122 studies are ongoing both as a single agent and in combination with other anti-lymphoma agents.
Ysebaert: Janssen: Consultancy, Research Funding, Speakers Bureau. Sancho: Janssen: Honoraria; Gilead: Honoraria; Laboratorios Servier: Consultancy; Celgene: Honoraria; Kern Pharma: Honoraria; Roche: Honoraria; Mundipharma: Honoraria. Salles: Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Cordoba: Foundation Jimenez Diaz University Hospital: Employment; Roche: Consultancy; AbbVie: Consultancy; Janssen: Consultancy; Gilead: Consultancy; Servier: Consultancy; Pfizer: Consultancy. Pinto: Merck Sharp Dome: Honoraria; Roche: Honoraria; Bristol Myers Squibb: Honoraria; Gilead: Honoraria; Celgene: Honoraria; Millenium Takeda: Research Funding; Helssin: Honoraria; Mundipharma EDO: Speakers Bureau. Gharibo: Bayer: Employment. Rasco: Celgene: Research Funding. Lopez-Martin: MSD: Consultancy; BMS: Consultancy; Novartis: Consultancy; Roche: Consultancy; Astra Zeneca: Consultancy; Celgene: Consultancy; Bayer: Consultancy; Pharma Mar Sau: Equity Ownership, Patents & Royalties: WO2008135793; Pfizer: Research Funding. Santoro: Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees. Salar: Janssen: Speakers Bureau; Roche: Speakers Bureau; Servier: Speakers Bureau. Damian: Fondazione IRCCS Istituto Nazionale dei Tumor: Consultancy. Martín: Janssen: Honoraria; Servier: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Gilead: Consultancy. Wei: Celgene Corporation: Employment, Equity Ownership. Hagner: Celgene: Employment, Equity Ownership. Hege: Celgene Corporation: Employment, Equity Ownership. Risueño: Celgene: Employment, Patents & Royalties: US15273205. Gandhi: Celgene Corporation: Employment, Equity Ownership. Buchholz: Celgene Corporation: Employment. Pourdehnad: Celgene Corporation: Employment, Equity Ownership. Ribrag: MSD: Honoraria; BMS: Honoraria; Gilead: Honoraria; Nanostring: Honoraria; Infinity: Honoraria; Roche: Honoraria; ArgenX: Research Funding; Servier: Consultancy, Honoraria; Epizyme: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal